Psilmi™ – Objective, AI‑driven digital biomarkers for psychiatry
Autism, ADHD, depression and anxiety.

Psilmi blends Ψ (Psi)—the long‑standing symbol of psychology—with Finnish silmä/silmiä (eye/eyes), reflecting both our scientific roots and our measurement focus.
What is Psilmi?
Psilmi is a patent‑pending software platform that uses structured visual stimuli and high‑precision eye tracking to capture objective physiological signals from the nervous system. Advanced modeling translates these signals into digital biomarkers intended to support clinical decision‑making—moving beyond behavior rating scales toward biology‑linked measures. The goal is offer earlier, more accurate diagnosis, when there are multiple co-occurring diagnoses and/or cases close to diagnostic cut-offs.
Regulatory note: Psilmi is currently used for research and evaluation within our beta program and is not a cleared medical device. FDA 510(k) submission is planned during 2026.
Clinical Signal
A recent university‑led pilot (n=178 high‑functioning adults) observed:
- Distinct pupillary regulation patterns
- Biomarker clusters correlating with diagnostic categories
- ~90% mean separability across conditions under study
Important: Pilot outcomes are preliminary and for research use. Psilmi is not yet intended to diagnose, cure, mitigate, treat, or prevent disease.
How it Works
Stimulus → Response → Modeling
- Targeted stimuli probe specific neuro‑cognitive functions.
- Ocular responses (e.g., pupil dynamics, saccades, fixation) are recorded via eye trackers under controlled conditions.
- Advanced models transform raw signals into interpretable biomarker features, designed to support differential assessment and longitudinal tracking.
Why it’s different: Instead of focusing on a single biomarker (such as autonomous regulation or social processes) and a single outcome (such as autism or anxiety) we build profiles using biomarkers from all dimensions in the RDoC and link it to multiple diagnoses (autism and anxiety and depression and ADHD, e.t.c.).
Join the Beta Program
Who it’s for
- Clinics and research centers in psychiatry, neurology, or developmental medicine
- Sites with access to eye‑tracking hardware and research governance (IRB/ethics)
What you get
- Access to the Psilmi Launcher and beta builds
- Onboarding, documentation, and integration support
- Participation in multi‑site protocol iterations and roadmap input
- Data tools for exporting study data (de‑identified if wanted)
Your commitments
- Follow site governance and consent processes
- Run defined stimulus protocols and calibration procedures
- Share de‑identified datasets and feedback on usability and reliability
Apply now
Send an email to psilmi@stardots.se and receive login instructions.
System & Device Notes
OS & Hardware: Modern Windows 11 computer. Dedicated GPU recommended
Eye trackers: Compatible with research‑grade devices via vendor SDKs. Most Tobii cameras supported if sample rate > 100 Hz.
Displays: Minimum 1920*1080 resolution. Minimum size 14″.
Network: For updates, login, license checks, and secure data sync and subsequent mathematical modelling.
Download
Windows x64 Installation (0.7.2 beta) MSIX
Instructions:
- Download the MSIX installation file above
- Double click the file to start the Psilmi installation process
Product Tour
We designed the Psilmi application with a modern aesthetics appeal—keeping the UI and UX easy and intuitive.
Psilmi Launcher
The Psilmi Launcher streamlines onboarding and maintenance across sites. It handles version checks and self‑updates, secure login, EULA acceptance, and the installation, updating and launching of Psilmi—reducing IT overhead and ensuring protocol consistency.
Key capabilities
- Splash & version control — verifies build integrity, checks for updates.
- Self‑update — applies signed updates before session start.
- Secure login — authenticates users per site policy (SSO/credentials).
- EULA & compliance — centralizes acceptance and version history.
- Install/Update & start — deploys or updates Psilmi, then launches into the app.

Psilmi Launcher initializing, showing version info and status messages.
Psilmi Medical Device
Home
- Overview and navigation
- Quick links to New Test and Analyze
Study
- Create and manage studies and participants
- Track inclusion criteria, site, cohort, and consent status
New Test
- Select Study, Participant, Stimuli, Eye Tracker, Monitor
- Add comments (context, protocol notes)
- Start, end with protocol guardrails
Analyze (in development)
- Preview of model outputs and feature summaries
- Export for statistical analysis / ML pipelines
Settings
- About
- Stimuli & Eye‑Tracker Management (libraries, calibration defaults)
- App Settings (UI, data paths, updates)
- Diagnostics & Console (logs, health checks)
- Feedback (feature requests, bug reports)

The Psilmi home screen
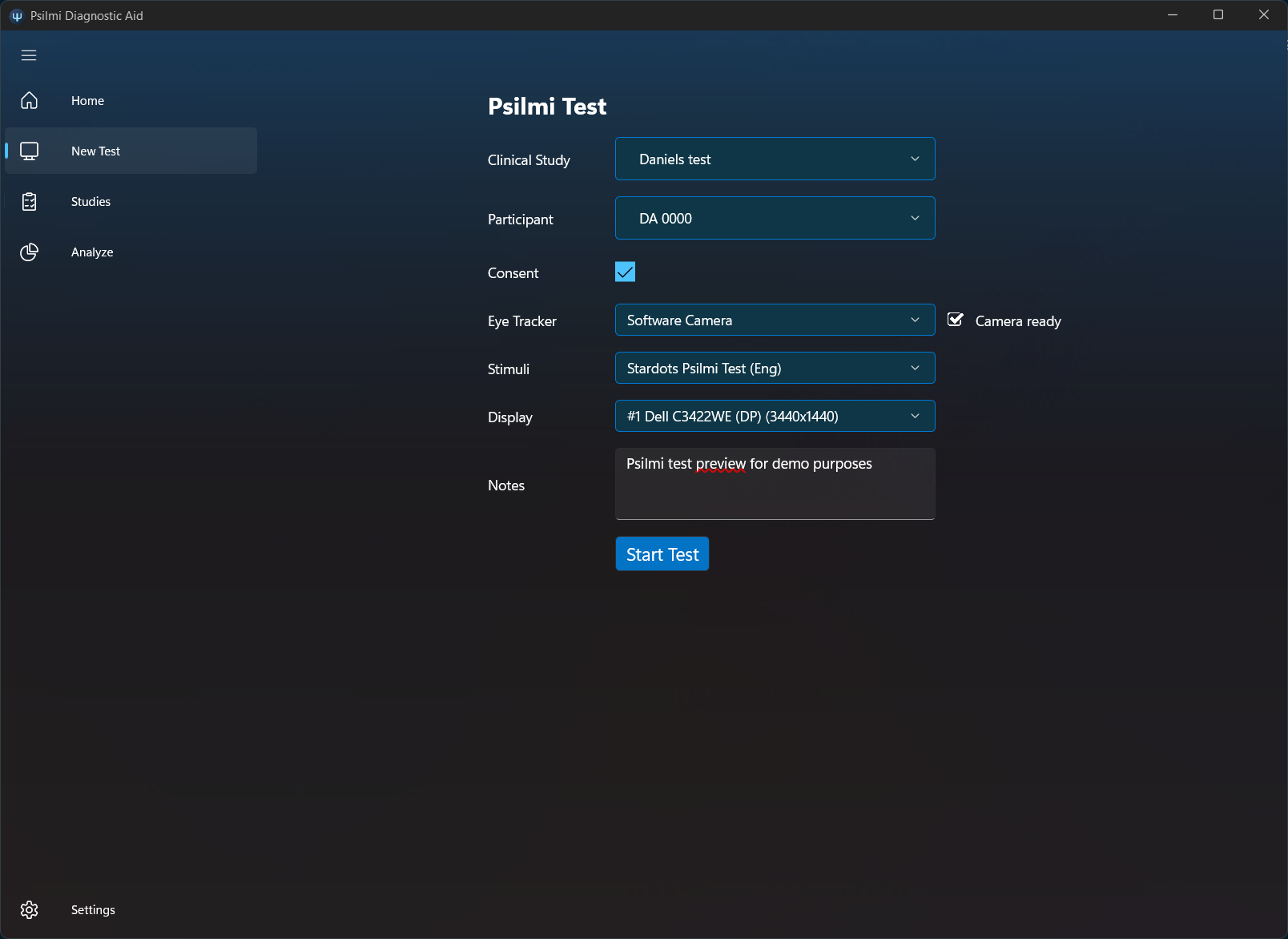
Start a new test by choosing study, participant, consent, stimuli, camera and display (if multiple displays are connected).
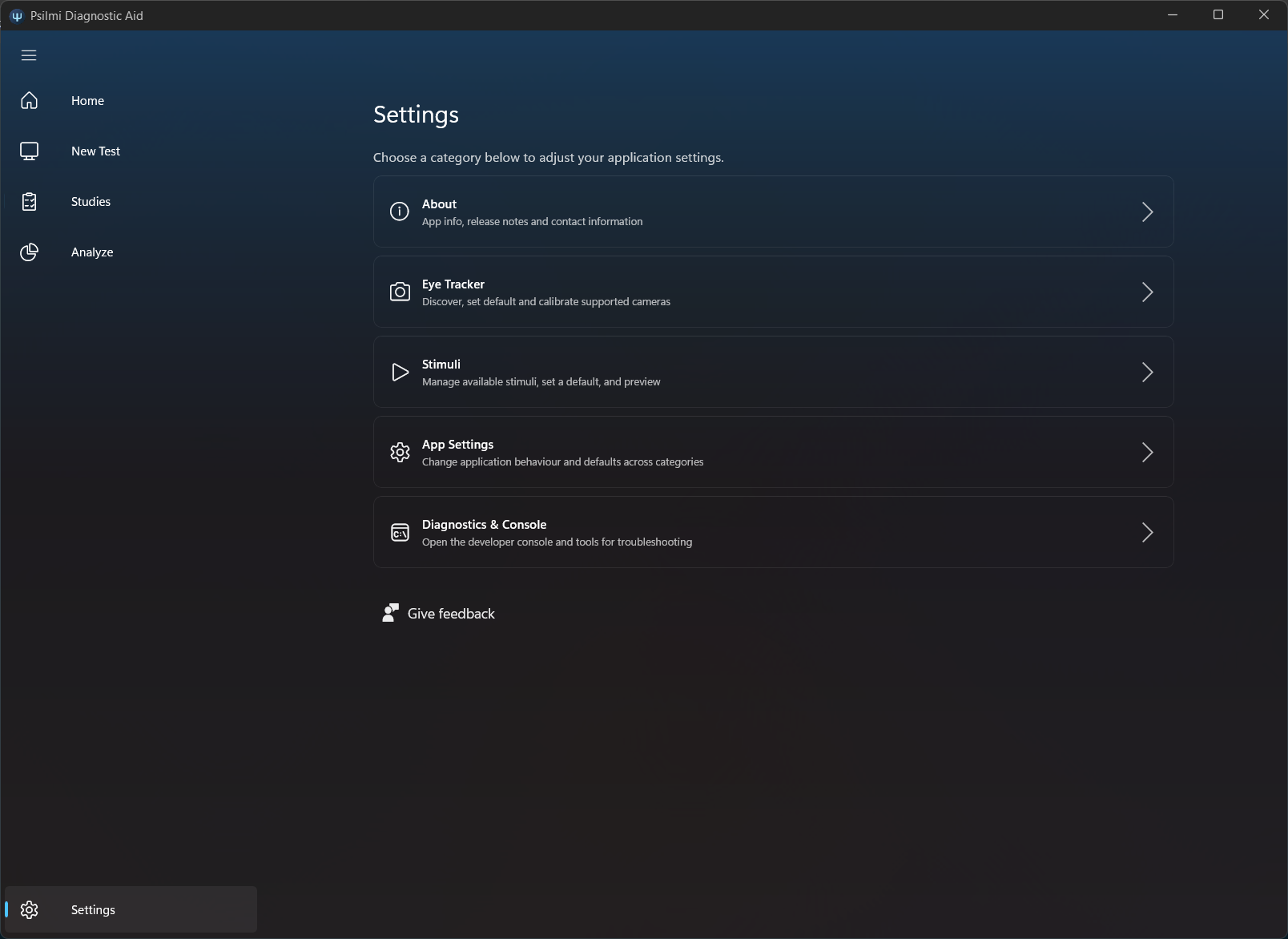
Settings controls every aspect of the Psilmi application including camera- and stimuli management, app settings, feedback and access the console.
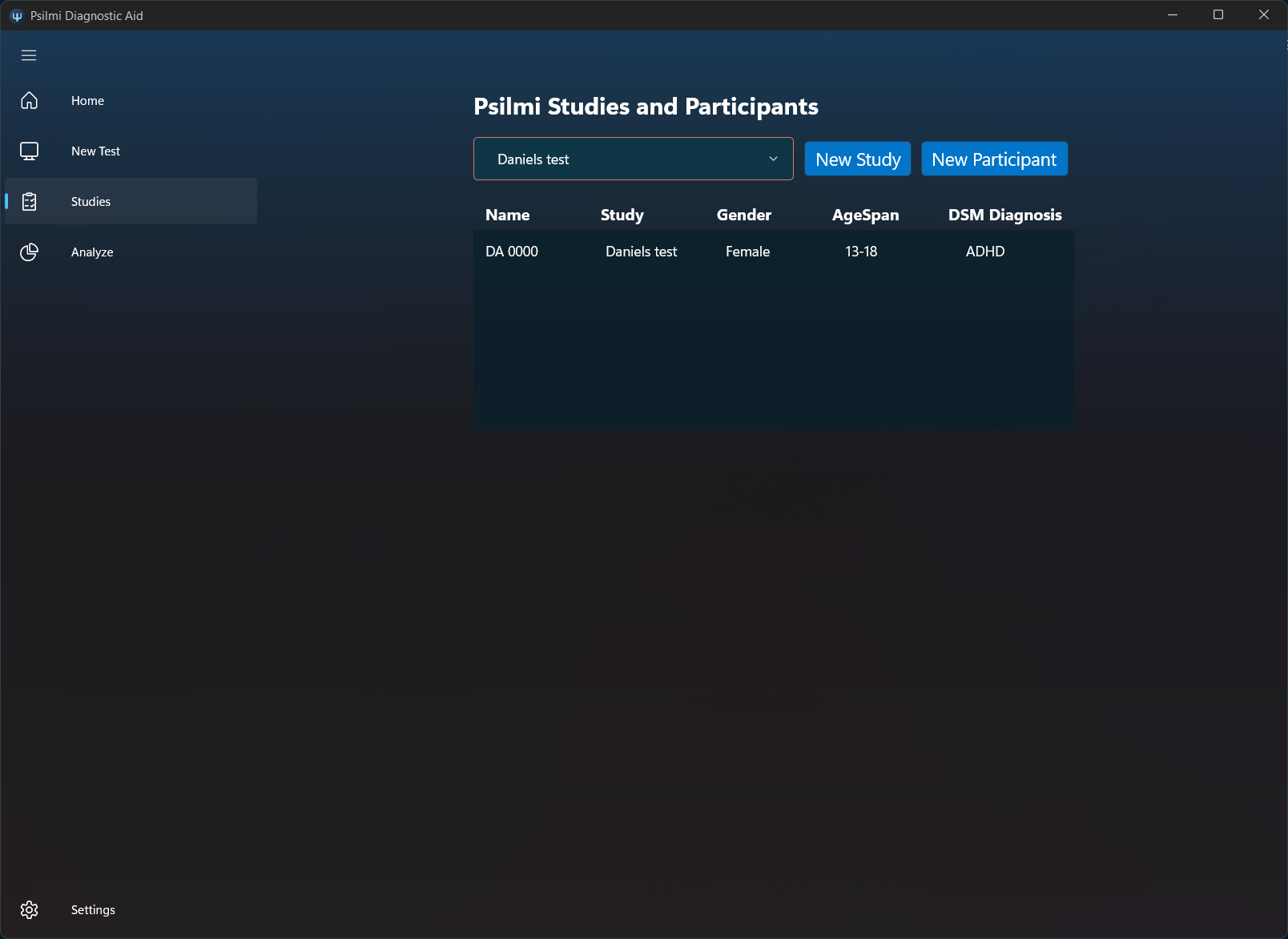
Create unlimited studies and participants including various demographics data and eventual diagnosis for quaility data input
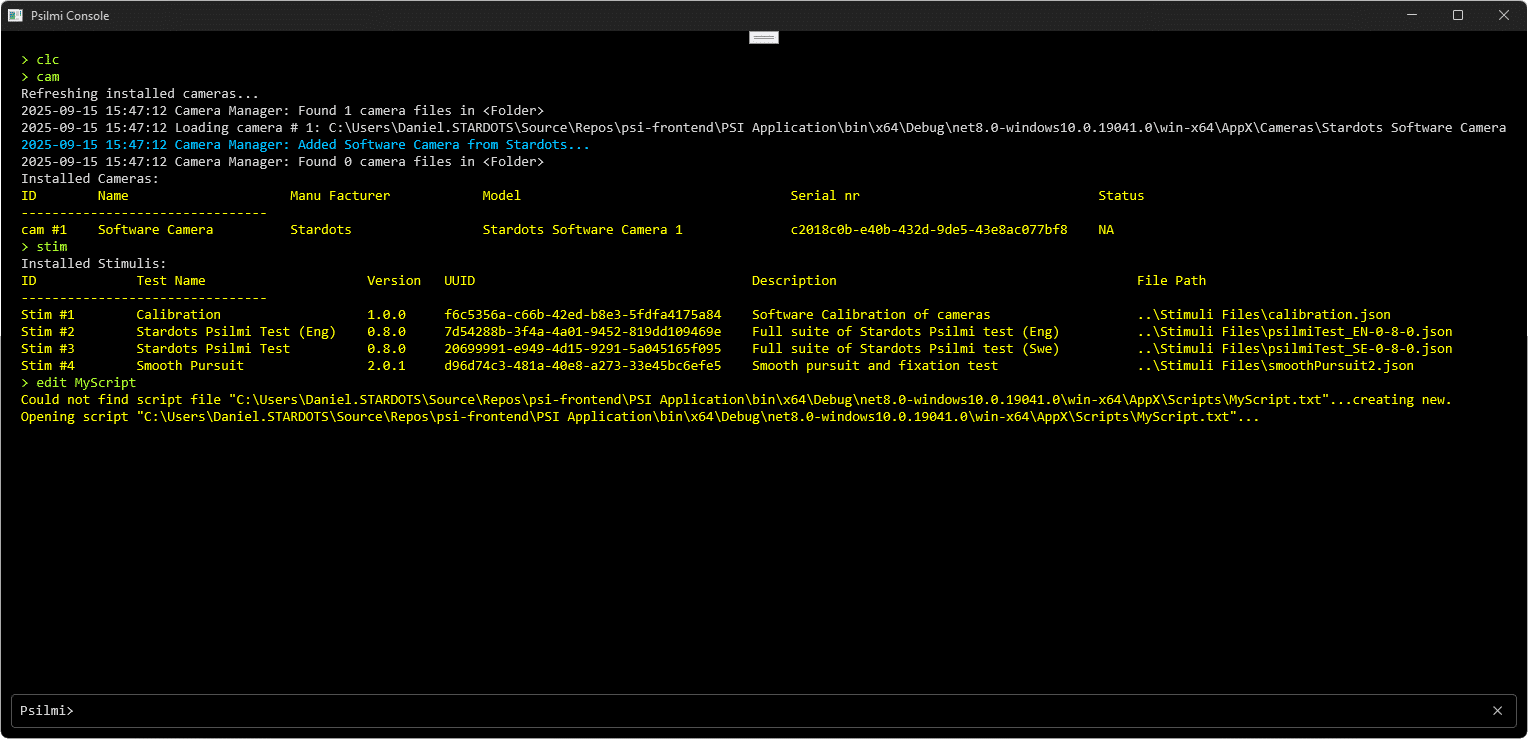
Psilmi features a rich console and the Psilmi-scripting language capable of complex instructions and automated work flows.
Security & Privacy
- Data ownership: Stays with the site and/or participant per study agreements
- De‑identification & pseudonymization options for exports
- Encryption in transit and at rest (implementation details available under NDA)
- Role‑based access and audit logs for study operations
- Optional offline capture with secure sync when connectivity is available
We support GDPR‑aligned practices and local ethics requirements; implementation specifics are finalized per site.
FAQ
Is Psilmi a medical device?
Not yet. It is currently provided for research use within our beta program. Regulatory submissions are planned.
What outcomes does Psilmi provide today?
Standardized acquisition of ocular physiology under controlled stimuli, with emerging biomarker features; analytical tooling is evolving.
How are participants consented?
Per site protocol and ethics approvals. Psilmi supports de‑identification workflows and data export controls.
Which eye trackers are supported?
We integrate with leading research‑grade systems via SDKs. Contact us for the current validated list, but most of Tobii eye trackers are supported, if the requirement of a minimum of 120 Hz sample rate is met.
Can we run offline?
Yes—offline test sessions with secure synchronization when connectivity returns (optional, configuration‑dependent).
Contact
Stardots AB, Uppsala, Sweden
Daniel Petrini, CEO, daniel.petrini@stardots.se, +46 70‑782 70 01
General inquiries info@stardots.se
Learn more www.stardots.se
Psilmi is co-developed with Uppsala University (Sweden) and aiming in first hand for FDA 510(k) US market clearance. Pilot study from 2024 shows strong diagnostic accuracy. Potential for expansion into more neuropsychiatric conditions.