ANLIVA® Hand movement
Parkinson’s disease
Parkinson’s disease (PD) is a neurodegenerative disease which worsens gradually over time and recurring clinical visits are required to manage the disease and track its progression. Most US patients typically see a neurologist 2-4 times per year, but limited availability of neurologists can lead to long waiting times. ANLIVA® Hand Movement aims to alleviate this by enable tracking of motor symptoms using a simple smartphone, giving patients and physicians objective insights and highly granular resolution into disease progression by providing several standardised tests which can be performed at any time. The app is designed for use in therapy fine-tuning, daily treatment, remote patient monitoring, and clinical trials.
- ANLIVA® Hand Movement provides objective quantifications of several standard motor tests including tests for different kinds of tremor and bradykinesia.
- Objective results from standardized tests can aid physicians and reduce the time needed for in-clinic visits.
- Making testing available to patients anytime and anywhere with no additional hardware requirements beyond their smartphone will greatly increase the resolution of disease progression and aid patients in their understanding of their disease.
- ANLIVA® Hand Movement enables patients to record standardized clinical motor tests at home.
- Beyond patients and physicians, objective results of standardized motor tests can provide insights and clear endpoints to pharmaceutical companies running clinical trials in the PD space.
Clinical trials in collaboration with Dartmouth-Hitchcock Clinic (USA) are underway, with FDA 510(k) clearance targeted for the end of 2025. Patent pending.
Our ANLIVA® Hand Movement medical device application is being developed with the aim of aiding patients and physicians in monitoring and characterising symptoms of movement disorders such as Parkinsons’ disease (PD) and Essential Tremor (ET) using a simple smartphone.
Currently, motor symptoms of PD and ET are evaluated by physicians during clinical visits typically occurring a couple of times a year. But these evaluations are both time-consuming and subjective as well as only providing updates on a patient’s condition at the time of visit.
The vision behind ANLIVA Hand Movement is to use the high-quality sensors present in modern day smartphones to allow patients to record the movements of standard clinical tests at any time of their choosing. Patients can choose to perform tests used to evaluate e.g. kinetic tremor, postural tremor, rest tremor or bradykinesia.
From the recorded sensor data, characteristic metrics of the movements are extracted which allow for the construction of objective measures of a patient’s movement disorder symptoms and provide a high resolution into the evolution of those symptoms. At the time of clinical visits, a physician can perform additional tests together with a patient and examine the history of results to gain a better understanding of the symptom progression.
The objective and standardized aspects of the results together with the result history also provides benefits to interactions with new physicians. Finally, the ANLIVA Hand Movement application also aims at providing a standard measure of symptoms relevant for any pharmaceutical company running trials with motor symptoms as part of their targeted endpoints.
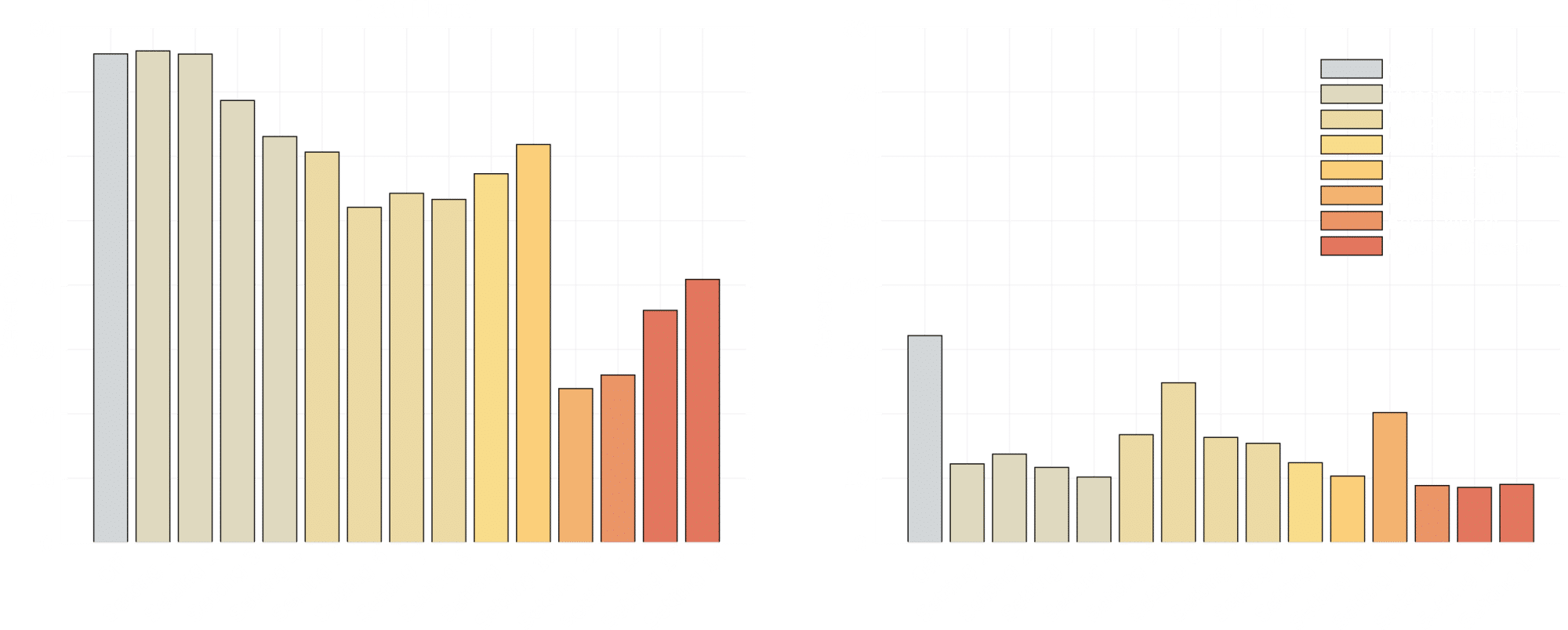
Results from an example case study is shown in figure 1 below where the ANLIVA® Hand Movement was used in titration of different settings for Deep Brain Stimulation (DBS) in a patient. A setting of the DBS was applied after which the patient performed a test using the application, then another setting was applied and so on, in total 14 different settings were evaluated. The severity assessment for each is shown for both hands, with a lower score indicating lesser symptoms. Overall, the best setting considering both hands is seen to be setting 12.