Stardots to initiate clinical study of ANLIVA DBS in Parkinson’s disease patients in collaboration with Dartmouth Hitchcock Medical Center
February 5th, 2025

Uppsala, Sweden – February 5 2025 – Stardots AB – a leading developer of software medical devices to simplify and improve the diagnosis and treatment of neurological and psychiatric disorders – today announces that it has broadened the collaboration with Dartmouth Hitchcock Medical Center in the U.S. to include the further development of ANLIVA® DBS, an innovative medical device software designed to optimize deep brain stimulation (DBS) treatment for Parkinson’s disease. Enrollment of patients in a clinical study of the device is expected to commence by mid-year 2025.
ANLIVA® DBS is a Software as Medical Device (SaMD) that employs sophisticated mathematical modeling to predict optimal DBS settings based on individual patient imaging data. The patent pending technology analyzes MRI and CT scans to generate patient-specific recommendations for DBS programming, potentially streamlining a process that traditionally requires multiple clinical visits over several months. ANLIVA® DBS has the potential to significantly improve treatment outcomes, enhance patient quality of life, and reduce healthcare costs associated with DBS programming.
“Following our already established collaboration with Dartmouth Hitchcock Medical Center in developing ANLIVA Hand Movement and ANLIVA Eye Movement, we are delighted to broaden the scope to include ANLIVA DBS. The aim is to evaluate the possibilities of optimizing deep brain stimulation to improve the quality of life for people living with Parkinson’s disease,” said Daniel Petrini, CEO of Stardots AB.
The proposed includes the initiation of an open, controlled exploratory study to evaluate the clinical efficacy of ANLIVA® DBS across three distinct patient groups: new DBS recipients preparing for initial programming, patients with satisfactory outcomes from current programming, and those experiencing suboptimal results from existing settings. Approximately 30 participants with Idiopathic Parkinson’s Disease will be enrolled. The study is expected to commence by mid-year 2025 and last 12 months.
“’There is a huge need for incorporating computerized selection and adjustment of DBS leads given the infinite possibilities that the neurologist has to deal with in reality of clinical practice. The current paradigm of adjusting DBS parameters is often based on a lengthy and manual trial-and-error procedure that depends on the subjective expertise of the clinician. Now that we started working on and planning for the clinical trial using ANLIVA DBS, that possibility became closer to actual application than ever.” comments Anas Hannoun, MD Clinical Assistant Professor of Neurology, Movement Disorders at Geisel School of Medicine at Dartmouth
The neurology clinic for Parkinson’s Disease and Movement Disorders of Dartmouth Hitchcock Medical Center, based in New Hampshire, U.S., has been designated a Center of Excellence by The Parkinson’s Foundation.
About ANLIVA® DBS
ANLIVA® DBS is a medical device software that predicts optimal deep brain stimulation (DBS) settings for individual patients, enhancing treatment effectiveness. Using digital twin technology, the software analyzes the patient’s neurological imaging data to determine optimal settings. This advancement replaces traditional manual adjustments, which burdens both patients and healthcare resources with time-consuming calibration sessions.
Healthcare providers simply upload patient imaging data to receive ANLIVA® DBS’s recommended settings. The software’s computational algorithms are drawn from extensive mathematical modeling research conducted at Uppsala University. FDA clearance through a 510(k) submission is anticipated in the first half of 2026.
For further information, please contact:
Daniel Petrini, CEO
Tel: +46 70-782 70 01
E-mail: daniel.petrini@stardots.se
About Stardots AB
Stardots AB develops software-based diagnostic tools (Software as Medical Device) for neurological and psychiatric disorders such as Parkinson’s, autism, ADHD, dementia and Alzheimer’s. The company’s product platforms ANLIVA®, PSI and QUEST use advanced mathematical modeling of sensor data from smartphones, eye cameras and other sensors to enable faster and more reliable diagnosis and optimized treatment. Stardots’ first product, ANLIVA® Hand Movement for Parkinson’s disease, currently being evaluated in a clinical trial in collaboration with Dartmouth Hitchcock Medical Center in the U.S., has the potential to reach market approval by the end of 2025. Stardots, headquartered in Uppsala, Sweden, has the U.S. as its primary target market.
For further information, visit www.stardots.se.
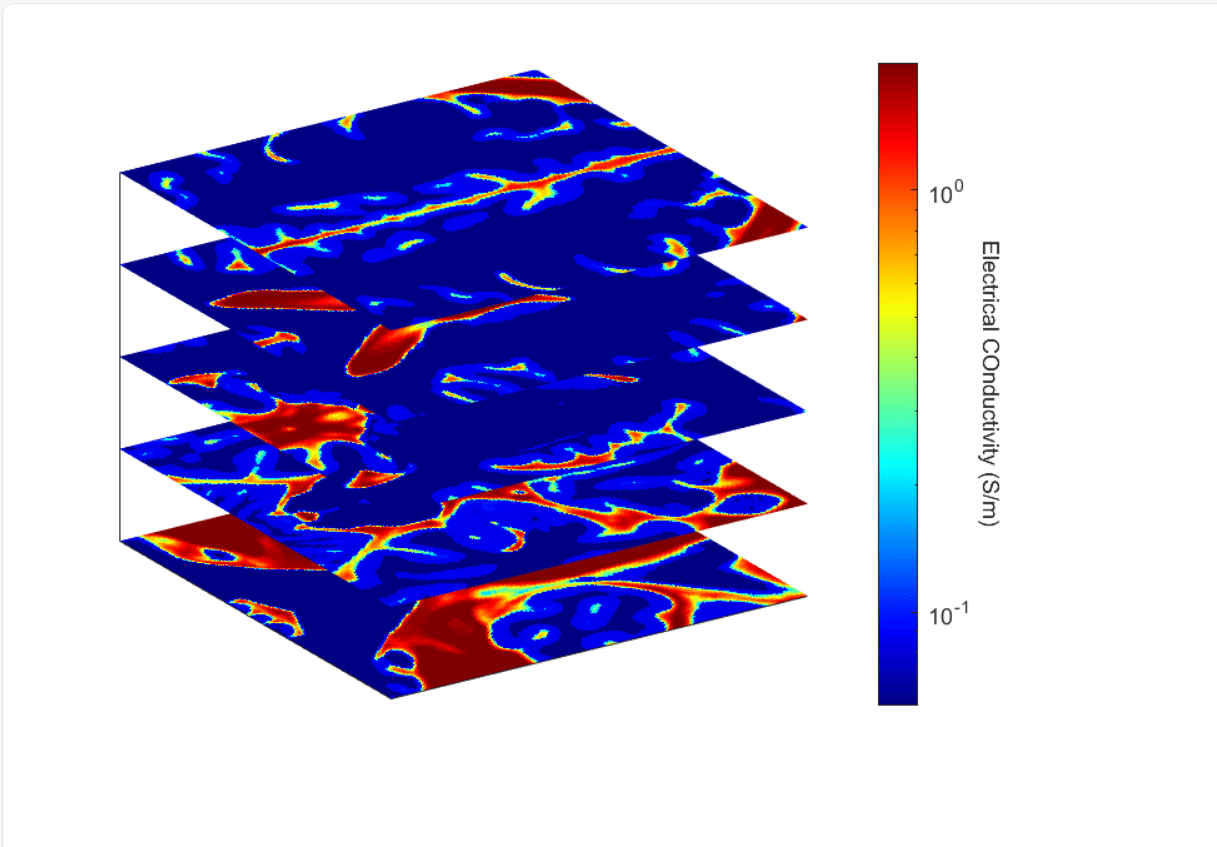
Intensity-based segmentation of an MR image to identify grey matter, white matter and cerebrospinal fluid is used to obtain patient-specific conductivity models for DBS simulation
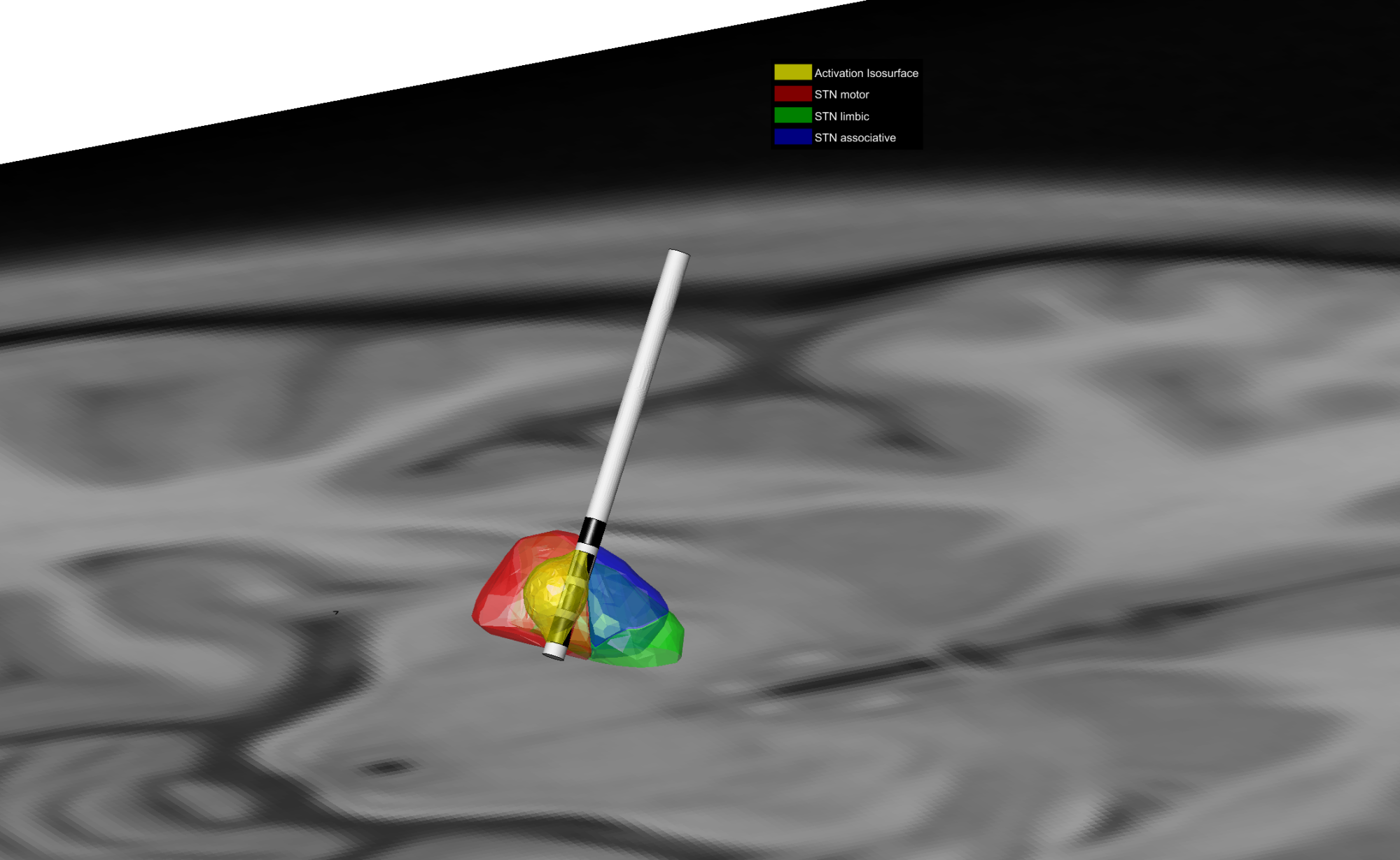
This image demonstrates the optimized activation field in Deep Brain Stimulation targeting the subthalamic nucleus (STN) motor region in Parkinson’s Disease (PD). By optimizing the electric field to precisely target the STN motor, the motor symptoms of PD are addressed while minimizing potential side-effects.